
Prepare and manage tracking reports of submission activities and other measures for department metrics.Provide administrative support to monitoring of regulatory submission timelines and meeting minutes.Assist in producing new or revised medical device submissions in the format consistent with the governing SOPs.
#Guideliner interventional registration#
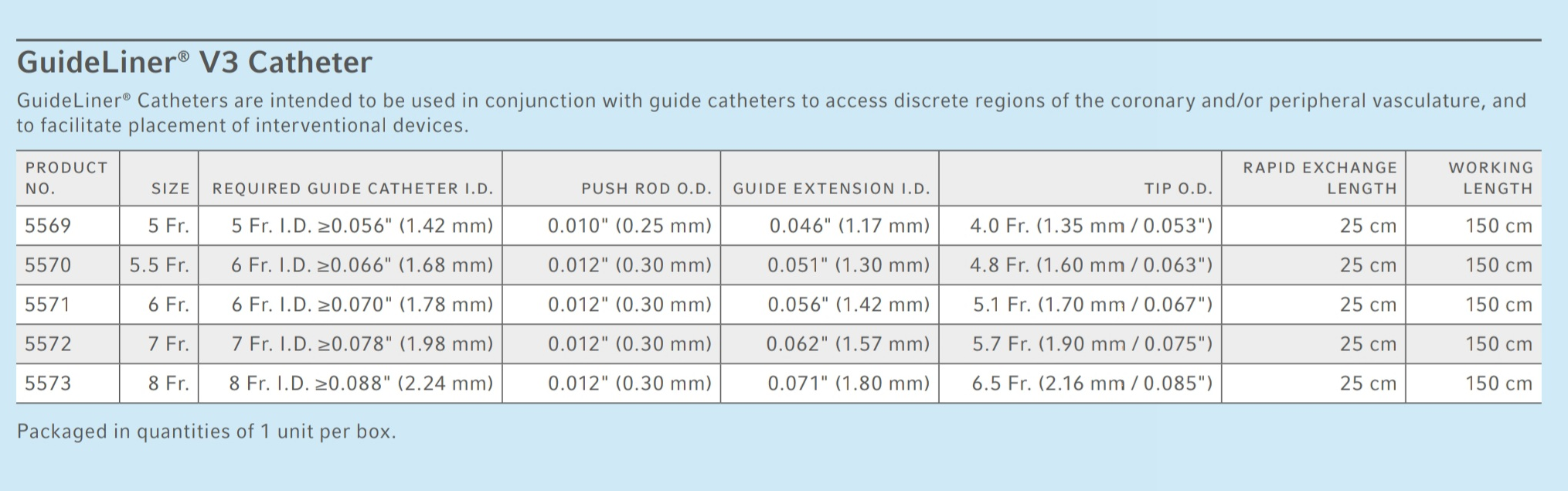
Our current Interventional products include a broad range of clinically relevant solutions, such as our GuideLiner® and Turnpike® Catheters, AC3 Optimus™ Intra-Aortic Balloon Pump and OnControl® Powered Bone Access System. We place a strategic emphasis on complex coronary and peripheral interventions, vascular access, bone access, specialty biologic treatments and cardiac assist. Interventional - The Interventional business unit at Teleflex offers innovative medical devices that are used to diagnose and treat coronary and peripheral vascular diseases. Teleflex employees worldwide are united in the understanding that what we do every day makes a difference. Our portfolio is diverse, with solutions in the fields of vascular and interventional access, interventional cardiology, surgical, anesthesia, cardiac care, interventional urology, urology, emergency medicine and respiratory care. We apply purpose driven innovation - a relentless pursuit of identifying unmet clinical needs - to benefit patients and healthcare providers. Expected Travel: Up to 10% Requisition ID: 6574 About Teleflex Incorporated Teleflex is a global provider of clinically effective medical technologies designed to improve the health and quality of people's lives.


 0 kommentar(er)
0 kommentar(er)
